Pistachio trees stand out among tree nut crops as tolerant of salty growing conditions, but they have their limits, and production may eventually be affected if steps are not taken to relieve stress that happens with salt accumulation. Pistachio orchards have been planted from the Sacramento Valley to Kern County as well as some desert locations. Estimates are that about 25 percent of pistachio acreage is salt-affected. Most of those orchards are in the southwestern San Joaquin Valley (SJV). Many areas have naturally high salt levels, as the soils are derived from sediment from the coastal range that was once below sea level. However, challenges with irrigation water quality and quantity have exacerbated the issue in recent decades.
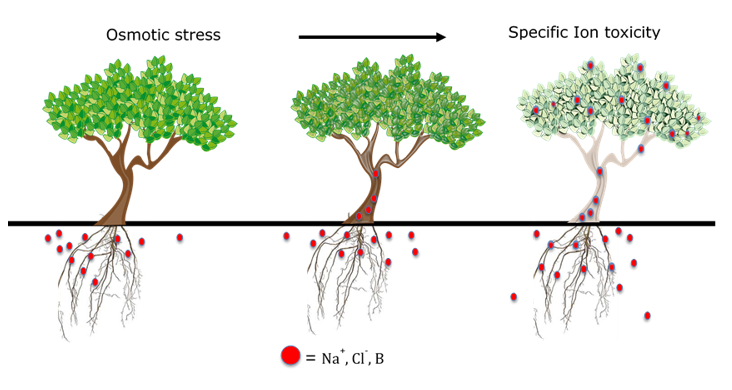
Depending on the severity, the harmful impacts of salt-affected soils can develop early in orchard establishment, resulting in stunted trees. Initially, trees are subjected to what is called osmotic stress, meaning they are working harder to take up and transpire water because of the increased salt concentration in the soil.
As salt concentrations increase around the roots, there is less solute differential between the root sap and the soil water. This reduces the ability of the root to pull in water by osmosis. As the salt pressure outside of the root continues to increase, it becomes too much for roots to exclude toxic salts and they begin to accumulate in the woody tissue (See Figure 1). Eventually, necrotic burns on the margins of leaves may result, a characteristic known as specific ion toxicity (See Figure 2). The composition of tree damaging salts varies across different agricultural areas, but problems commonly result when high levels of sodium (Na), chloride (Cl), boron (B), and bicarbonate (HCO3) are present in the soil and or water alone or in combination with each other (Figure 1). Sodium is among the most problematic of these salts because in addition to the problems caused by osmotic stress and ion toxicity, high levels can also degrade soil structure and reduce drainage from the orchard floor, further compounding tree root stress.
Salt affected soils are commonly referred to as ‘saline’, but it is important to know the distinction between three broad categories of salt-affected soils: saline, saline-sodic and sodic soils. Saline soils have high overall soluble salt concentrations, but usually have higher levels of soluble calcium, which minimizes problems with sodium. These soils generally have good soil physical characteristics and can be managed with sufficient leaching water and careful fertility management, without the addition of amendments. Saline-sodic and sodic soils can have varying levels of overall soluble salts, but high levels of Na in either situation. These soils require soil and water amendments to improve soil physical conditions to effectively leach Na out of the soil.

What to Look for from Soil and Water Reports
High salt levels must be decreased below toxic thresholds to maintain tree growth and production. Knowledge of orchard soil and water conditions is critical to ensure salts are managed appropriately. Laboratory reports provide a lot of information and it can be difficult to know how to interpret them to make management decisions. A basic understanding of soil type and cation exchange capacity (CEC), overall soluble salts, pH in soil, and water can help to identify problems and make informed decisions about how to manage them. Electrical conductivity (EC), reported as either deci-Siemens per meter (dS/m) or millimhos per centimeter (mmhos/cm), is a measure of total dissolved salts present in irrigation water or soil water that provides an indication of potential for salt stress on tree growth. Both units have the same value.
Pistachios are salt-tolerant, but depending on the salt composition, irrigation water exceeding 4.5 to 7 dS/m EC is probably not sustainable for the long term, especially if salinity challenges are coupled with poor soil drainage. Salts are either positively charged cations or negatively charged anions that have varying levels of impact on overall salinity stress, with more or less effort to manage. Soil chloride (Cl) and sodium (Na) levels >30 milliequivalents per liter (meq/L), and B levels above 3 mg/L are problematic for the long-term health and productivity of pistachios. Cl can be reduced with clean water alone, however B ions strongly hold (adsorb) to soil particles at higher pH levels, and acid amendments with ample water are necessary to flush excess B out of the root zone. Na requires continual management to mitigate current or future problems with toxicity and declining soil structural stability and drainage. However, sufficient levels of calcium (Ca) and magnesium (Mg) can counter or reduce the severity of its effects. The sodium absorption ratio (SAR) and exchangeable sodium percentages (ESP) are indices used to compare the concentration of Na with respect to Ca and Mg levels. A general rule of thumb is SAR levels greater than five times the EC of irrigation water indicates an imbalance of Na to Ca and Mg levels, and likely problems with infiltration.
Elevated pH greater than 7.5, bicarbonate (HCO3) greater than 2 meq/L, and soil lime (CaCO3) > 1% indicate potential for reduced nutrient availability, as well as soil sealing and reduced infiltration. High HCO3 levels appear as a white chalky substance on the soil surface and micro-irrigation emitters, which alone can be a major source of clogging when not amended properly. When both Na and HCO3 salts are present, HCO3 can tie up Ca in the soil and water, which allows Na to become the dominant cation on soil particle surfaces in the formation of saline-sodic or sodic soil conditions. Calcium is a positively charged ion that is attracted to negatively charged soil clay particles, this attraction aggregates soil particles, giving soil structure that resists dispersal and degradation. Unlike Ca, Na ions cause spaces between soil particles to swell, and at elevated levels will eventually disperse soil aggregates. When loose particles clog the pores and cracks that usually allow soil water to infiltrate into the ground, impermeable crusts can develop on the surface. These crusts slow or stop water infiltration and decrease oxygen levels needed by tree feeder roots to absorb nutrients. These saline-sodic or sodic conditions require soil and or water amendments to improve soil structure and leaching for better tree health.
Soil and Water Amendments
The purpose of amendments is to provide a source of Ca to replace the Na and remove it from the root zone. This can be accomplished with either the direct application of soluble Ca (usually as gypsum) or by use of acidifying products that react with Ca bound by HCO3 or soil lime (CaCO3) to make the Ca ion soluble. A combination of both amendments can be beneficial. The sulfate binds with Na to form a compound that is easily leached from the soil, while Ca takes its place on the surface of soil particles. A large quantity of Ca is generally needed to displace Na. Yet, because amendments add salts to the soil, too much at once can add to osmotic stress that limits water uptake by trees. Also, any excess applied Ca not displacing Na, has potential to precipitate to form lime or even leach with the irrigation water dependent on soil conditions. The right amendment or combination of amendments largely depends on soil type, pH, the balance of Na with Ca and Mg, and whether there are appreciable amounts of HCO3 and CaCO3 in the water and soil.
Calcium amendments are most useful to displace Na where soil pH is less than 7.5, native calcite or lime is less than 1%, and in areas with ultrapure low EC (<0.2) water. The most common Ca supplying product, gypsum, is valued for its moderately slow release of soluble calcium (24%) to continually improve infiltration and provide a source of plant nutrients. However, solubilities or release rates vary dependent on particle size. Finer particle size products have better solubility and will work more quickly and predictably than coarse material. Fine, solution-grade gypsum injected as a slurry into micro irrigation systems is the most common application form used in-season. Before injecting, managers should evaluate if bicarbonate levels are high (>2 meq/L HCO3), as injection may cause precipitation of lime and clog irrigation lines. For dormant season applications, growers may consider using coarser sources such as ground wallboard. The larger particle-sized material will slowly dissolve with repeated irrigations throughout the season. Not all sources are equal in terms of purity. Ask your retailer for the bulk percentage of soluble calcium in the product. A lower grade with less Ca is generally more economical but will require heavier application to achieve satisfactory results.
Acidifying amendments are generally most useful in sodic soils with a high pH with soil lime levels greater than 1%. High levels of HCO3 can also be effectively neutralized by some acids. If soils have less than 1% lime, Ca must be added with the acid to create gypsum in the soil for Na to be leached. However, before these processes can take place, acid-forming amendments (for example sulfur, ammonium polysulfides, and thiosulfate) require an initial biochemical oxidation of sulfides (S2) by bacteria, named Thiobacillus, to form the sulfuric acid that then breaks down the lime to form free Ca and gypsum. This is important to note because soil temperature and moisture greatly influence microbial activity and the successful conversion of the acid amendment to gypsum. Moist soil with temperatures above 55°F for a period of one to two weeks is needed for the reaction to take place. Optimal soil conditions are around 80°F, therefore a fall application of sulfur will likely provide Na leaching benefits the following growing season rather than during the dormant period when it is commonly applied. Sulfur products, like gypsum, are available in a variety of particle sizes. The finest sized particles provide the fastest response for gypsum formation, while gravel-sized particles (popcorn sulfur) can take many years to completely react in the soil.
Sulfuric acid and other sulfate-based liquid acids like urea sulfate make gypsum in the presence of a lime source, independent of the temperature and moisture conditions and do not require microbial oxidation. In addition to the removal of Na, sulfuric acid assists in dissolution of HCO3 in highly sodic soil and irrigation water. Sulfurous acid, a safer alternative to sulfuric, is generated with a sulfur burner, which is then injected into irrigation water. Sulfurous acid may provide longer residual acidity that continues to neutralize HCO3 and form gypsum.
The use of organic matter including composts, mulches and cover crops are a final consideration in soil amendment goals. Organic matter enhances microbial activity leading to soil aggregation and improved infiltration and can enhance the effectiveness of other amendments. Kern County Emeritus Soil and Irrigation Advisor, Blake Sanden, found mixing 1 ton of fine-ground soil sulfur with 4 tons biosolids compost and side banding over the drip hose (a 3” thick by 2-foot wide band) with a low salt source of well water successfully improved water infiltration in Eastside orchards with severe soil sealing problems. Composts and mulches can also be sources of salt (Figure 3), which can accumulate with salts from irrigation water, therefore laboratory analysis of these materials is recommended before use. Ongoing research is investigating whether additional surfactants, polymers, biostimulants and nutrient amendments reduce soil salinity and improve tree growth and yield in pistachio and almond trials. Cover crops have been shown to improve water infiltration, protect the soil from surface crusting, and in some instances, significantly reduce soil Na levels. Current research is looking at the benefits and tradeoffs associated with their use in established pistachio blocks.

Rates and Timing
Determining whether to amend irrigation water, the soil, or a combination of both, is dependent on the type and depth of the salt problem. If accumulating salts are limited to the shallow soil surface and not dominated by Na, water amendments applied in frequent small amounts by drip or micro-irrigation may be all that is necessary to address the issue. The N fertilizer content of some acid-forming amendments may be sufficient and more economical than acid injection to correct the formation of soil sealing lime at the surface. Conversely, if Na loads are high throughout the root zone, banded or injected acid and or Ca supplying soil amendments may be needed in addition to other amendments applied to the water. To determine if salt accumulation is mainly at the surface or through the root zone, soil samples should be taken from zero to three inches, then in one-foot increments, down through the root zone to four or five feet. Poor irrigation water quality with high Na (>15 meq/l Eastside soils, >30 meq/L Westside), on poorly structured soils, will require a focused seasonal to continuous amendment strategy to mitigate current or future problems. Some westside soil types (Panoche, Buttonwillow, Belridge and others) where drainage is not limiting do a better job of handling sodic irrigation water.
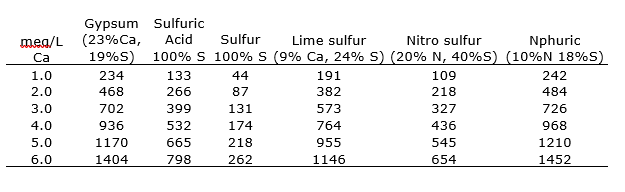
If applying amendments through water, determine the rate based on how much Ca (meq/L) or lbs. of material per acre-foot of water is needed to raise the water EC so that SAR is no more than five times the EC (Table 1). A typical in-season injection rate for fine, evenly graded, 100% gypsum would be between 200-300 pounds per acre, applied monthly in June, July and August. Typical acid injections range between 400-500 lbs. per acre-foot of water. Step by step instructions for calculating water applied rates of can be found in the UCANR publication 3375 Agricultural Salinity and Drainage p. 113-115, and in the UCANR publication 3545 Pistachio Production Manual p. 148-149, or at anrcatalog.ucanr.edu.
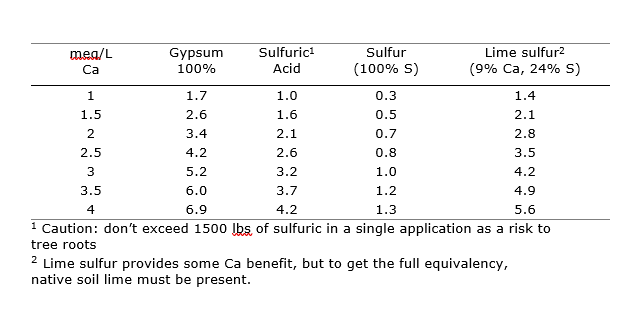
If soil reclamation is necessary throughout the root zone, larger amounts of soil-applied materials can be used in a single application (Table 2). Use the SAR, exchangeable sodium percentage (ESP), and CEC from your soil and water report to determine the Ca requirement and corresponding amendment rate for different materials. Calculation examples can be found in UCANR publication 3375 Agricultural Salinity and Drainage p.116-118. Banding gypsum along drip lines or micro-sprinklers is more cost-effective than full-field broadcasting. Smaller amounts of acid or fine sulfur, shanked into the soil along the drip hose, may increase the efficiency of applied amendments. Acid banding on berms in established orchards should not exceed 1,500 pounds per acre to avoid tree damage. These strategies are often easiest and most effective when done just prior to planting (Figure 4). If in-season applications are not sufficient to reduce sodic conditions, a dormant season broadcast application of a coarser, slower dissolving amendment is recommended. If reclaiming a highly sodic site prior to orchard establishment, 3-6 tons of gypsum should be mixed to as deep as the expected root zone or to at least 12 inches (30 cm) with a final surface application prior to establishment.
Leaching Salts from the Rootzone
Leaching salts from the root zone can be effective in preventing salt build-up in soils. The aim of dormant season salinity management is to replenish soil moisture, improve water penetration and leach enough salt for efficient use of irrigation water during the growing season. Prior to leaching, water and soil sampling and analyses should be conducted around early November.
For saline-sodic and sodic soils, make dormant gypsum amendment applications in the fall prior to rain and leaching. Depending on the soil texture and salt load, 6-10 inches of effective rainfall (penetrating past a 2” depth) or fresh-water winter irrigation is needed for efficient leaching after the appropriate amendments have been applied. Leaching calculation and conversions can be found at cekern.ucanr.edu.
If leaching with fresh irrigation water, the first application should fill the soil profile to field capacity. Allow two to four days for drainage, then begin leaching applications. Irrigators should make sure to keep irrigation sets to less than 24 hours to avoid the risk of soil saturation and Phytopthora. A good strategy is to begin applying water in January (one inch per event) leaving the soil some ability to absorb any rainfall that occurs before flowering. If no additional rainfall occurs, continue applying one inch every few weeks with the goal of reaching the target 6-10 inches before March. Soil and irrigation water should be sampled again to determine effectiveness of the leaching and provide a reference point for the salt levels at the beginning and end of the growing season.

Managing Salts in Summary
Pistachios are more tolerant than other tree crops, but elevated salt degrades soil structure, decreases water uptake, stunts growth, eventually accumulates salt in tissues and decreases nut crop quality. Knowledge of soil and water conditions is critical to selecting appropriate Ca supplying and acidifying products to mitigate these issues. Calcium supplying amendments are best for use in Na affected soils where lime levels are below 1% and HCO3 is lower than 2 meq/L. Acidifying amendments are useful in sodic soils with soil lime levels greater than 1%. Elemental sulfur and sulfide containing amendments require warm moist soil conditions for successful microbial oxidation to sulfuric acid, which dissolves soil lime, forms gypsum and removes Na. In general, injected amendments treat shallow sodic surface conditions, and soil applications address rootzone problems. Use caution not to over-apply amendments, including composts and mulches that might contain high levels of salt. Apply amendments prior to leaching reclamation efforts during the dormant season. Do not saturate the soil during leaching events. Limit winter irrigation sets to less than 24 hours, then allow soil to drain for two to four days between applications. Finish leaching applications at least two weeks prior to bud break in the spring.