In California, infection of almond by diseases starts at bloom time and continues with infections of the young green fruit and the fruit at the hull split stage. Wet conditions in late winter/spring favor infection of almond blossoms by brown rot fungi, such as brown rot (Monilinia fructicola), anthracnose (Colletotrichum acutatum) and gray mold (Botrytis cinerea). Also, bacterial diseases, such as bacterial blast (Pseudomonas syringae) and bacterial spot (Xanthomonas arboricola pav. pruni) can infect blossoms and leaves of almond. The young green fruit, if weather conditions are favorable, can be infected by pathogens such as Botrytis cinerea, Sclerotinia sclerotiorum, Collectorichum acutatum, Fusicladium carpophilum and Alternaria alternata. The third stage of susceptibility is when almond hulls split and the fungi that infect the hulls cause hull rot.
Hull Rot
Hull rot is not a new disease of almond. This disease has been reported several times in the past and it has become an annual and serious problem in almond orchards in recent years. The causal agents initially were determined as two fungi, mainly the bread mold fungus (Rhizopus stolonifer) and the brown rot fungus (Monilinia fructicola). Previous studies showed that hull rot in almonds grown in the Sacramento Valley had high incidence of Monilinia, while hull rot in the rest of the state was mainly caused by Rhizopus stolonifer and occasionally by Monilinia. There were also cases where both fungal pathogens could be found in the same orchard causing hull rot. With changes in cultural practices and the denser plantings of almonds, vigorous rootstocks that boost the growth and the general vigor of almond cultivars, intense fertilization and sufficient irrigation to satisfy the crop’s requirements, hull rot has become a major disease that is very difficult to control, and the almond industry has put forth tremendous efforts and supports research to find ways to manage it. In addition to the difficulties in controlling hull rot, we are finding new fungal foes attacking almonds and causing hull rot.
In the last several years, almond growers, farm advisors and PCAs noticed that the incidence of hull rot has increased to very high levels, resulting in major economic losses, and this is because a) the nuts with hull rot do not shake easily (stick tights) and frequently they will require a second shake; and b) large numbers of nuts can remain on the trees during the winter months as mummies serving as sites for the overwintering of navel orangeworm (NOW). Thus, to achieve a successful sanitation as recommended for reducing NOW damage, extra efforts by the grower and greater costs are required.
The unusually high levels of hull rot prompted growers, farm advisors and PCAs to start submitting to our lab (Kearney Agricultural Research and Extension Center in Parlier) an unusually high number of samples with hull rot and requesting whether we were dealing with more fungal pathogens in addition to the usual mentioned above as the cause of hull rot.
The suspicions of farm advisors and growers were proven to be founded. In diagnosing hull rot, one can examine the inner surface of the hulls after they have split and determine the mycelia and sporulation structures the pathogens develop. For instance, if there were whitish cottony mycelia with black peppery structures giving the appearance of gray cottony fussy mass, these would be the mycelia and sporulation of Rhizopus stolonifer (Figure 1). If there were buff color sporulation in a defined area (lesion) internally corresponding to a beige color lesion on the outside of the hull, this would suggest infection by the brown rot pathogen, Monilinia fructicola.
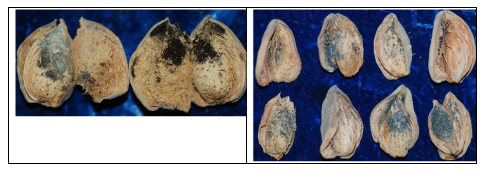
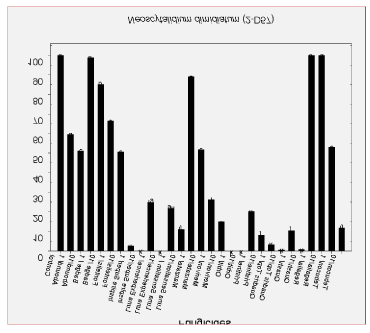
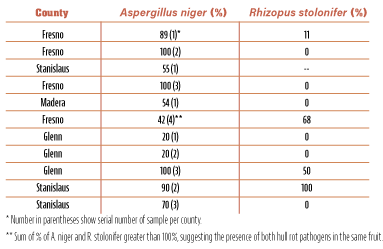
Lately, though, examination of almonds with hull rot symptoms using a hand lens and/or a dissecting microspore in the laboratory showed another type of sporulation between the hulls and shell. This sporulation was black and shiny in color and did not show the gray appearance that characterized hull rot caused by Rhizopus stolonifer. This black and shiny sporulation belongs to Aspergillus niger (Figure 1), a fungus that is very common in soils of nut tree orchards. In fact, analyses of many samples indicated that, depending on the particular orchard, there were cases in which samples had very high levels of A. niger, alone or in combination with R. stolonifer in the same fruit (Table 1).
Similar survey results were found in samples collected in 2016, 2017, 2019 and some in 2020.
Case #1: Kern County
In December 2014, farm advisor emeritus for Kern County Mario Viveros presented a severe case of hull rot associated also with excessive shoot and branch dieback that developed unusually large gum galls. Nuts still hanging on the trees (stick tights) were taken from four varieties: Nonpareil, Independence, Fritz and Monterey. Independence had the most and Monterey the least “gummy” nuts at the peduncle. Fritz and Nonpareil were between the two extremes in appearance of gummy nuts. Gummy nuts are the ones that show gum between nut and peduncle which results in sticktights, making harvest expensive (shaking trees twice) and difficult. Arrangements were made to collect and analyze some of the sticktights and also shoots that showed the discoloration with the large-sized gum galls (Figure 2A). It was apparent the infections from the fruit moved via the peduncle to the spur and then to last year’s shoot down to older shoots (Figure 2B). Results of isolation incidence from those samples are presented in Table 2.

The results from these first analyses were very surprising because a new plant pathogen was isolated from the fruit, shoots and peduncles. The pathogen consistently isolated at very high levels from all of these tissues and branches was a member of the fungal family Botryosphaeriaceae, now called Neoscytalidium dimitiatum. This fungus was reported with the name Hendersonula toruloidea in California in about the middle of the 19th century as the cause of a branch wilt disease of walnut. Taxonomists for some time changed the name from H. toluloidea to Nattrassia mangifera, and in recent years to Neoscytalidium dimitiatum. As soon as we isolated the fungus, we inoculated detached fruit and almond shoots. Following these inoculations, we inoculated fruit on trees to determine whether hull rot will develop leading to spur and shoot killing. All the detached fruit were infected with lesion formation three to four days after inoculation and were covered with dark greenish sporulation of N. dimitiatum 10 days after inoculation (Figure 2).
Arrangements were made with the help of PCA Chris Cucuk to visit these orchards where the mummy nuts and the shoots with gum galls were collected in the new growing season (2015). A visit in July was worthwhile because old and new severe symptoms were apparent, and the damage was characterized as severe. Interestingly, the abundant nuts with hull rot showed a black sporulation inside the hulls (Figure 1) that had a greenish hue and looked very different from the sporulation of the bread mold fungus or the dark shiny black sporulation, characteristic of that caused by Aspergillus niger. Up to 10 shoots were collected from four representative trees of each Nonpareil and Independence that showed severe symptoms, with the large gum galls still on the shoots.
The shoots were cut crosswise and the bark was scraped to reveal the discolored internal tissues (Figure 2B). Isolations were performed by cutting the discolored tissues to pieces of 4 x 5 x 4 mm and cutting the peduncle in half, and the surface sterilized in a 10% chlorine (bleach) solution and plated on acidified potato-dextrose agar media. Results revealed high levels of N. dimitiatum from both the peduncles and the cankered tissues (Table 2).
By scrapping the shoots and branches, it was apparent that infections from the nut moved through the spur down to last year’s shoot and from the shoot to older wood. The fungus was isolated from all these cankers, and about four inches below the lower margin of cankered tissues (Figure 3).

Although this was an extreme situation of very high incidence of infection of almond fruit with N. dimitiatum, it became apparent that when inoculum was abundant in the area and the almond fruit were at a developmental stage very susceptible to this pathogen (early hull split and even earlier stages), N. dimitiaum can cause hull rot and, furthermore, can kill spurs and shoots with devastating results during the current and next production years.
Case #2: Madera County
In 2019, Phoebe Gordon, UCCE farm advisor in Madera and Merced counties, had a grower in Madera with very severe hull rot in his almond orchard, and he needed help to figure out why there was so much shoot killing (Figure 4). Indeed, a visit to the orchard revealed a very high incidence of blighted shoots with blighted leaves attached to the killed spurs and shoots. The blighted spurs with the attached leaves were very similar with the hull rot symptoms caused by R. stolonifer; however, no characteristic gray mycelia and sporulation of Rhizopus were present under the hulls (Figure 5). Instead, there was white mycelia growing on top of the shell. It was apparent that the killing (blighting) of the shoots was advancing from the fruit to peduncle, spurs and shoots, resulting in blighting of large sections of shoots. Therefore, samples were collected for isolation of the fungus occurring in the nuts, and blighted shoots for dissection, observation and isolation.
Isolations from the nuts showed 56% Neoscytalidium on its own while of the fruit had Neoscytalidium and Rhizopus, and a very small percentage (<10%) had only Rhizopus. On the northeast side of the almond orchard, there was a large fig orchard. Some of the trees had obvious symptoms of limb dieback. Limb dieback of fig is caused by Neoscytalidium dimitiatum (see Progressive Crop Consultant, March/April 2021, pp. 24-29). Once we found out that the almond orchard was loaded with hull rot caused by N. dimitiatum, we tried to find out where this inoculum was coming from. We questioned the grower who admitted that a couple of weeks before we observed the severe Neoscytalidium hull rot symptoms, he had pruned the dead limbs due to limb dieback and then all the prunings on the ground were shredded. This is an ideal situation for spreading inoculum of N. dimitiatum because this fungus after infection of limbs converts all its mycelia upon maturation to dark, minute spores which formed between the woody tissues and the bark.

As the bark separates from the woody tissues, the shredding of prunings liberates the spores that then can easily become airborne (Figure 6). Isolations from the figs next to the almonds in Madera County produced almost 100% N. dimitiatum, which morphologically looked the same as the one isolated from the almonds. Although, at the time the almonds were collected, the mycelia were white inside, and after keeping these nuts for a couple of weeks in the laboratory, the white mycelia turned to greenish/black, very typical of the fungus N. dimitiatum. Also, isolations from figs with limb dieback in this orchard next to the almonds with the severe hull rot revealed high levels of N. dimitiatum, similar to the one isolated from the almonds. Therefore, this situation resembles the one at Kern County where the severe infection of almonds, which were at the right hull split stage, was the result of severe infection due to abundant inoculum formed after grinding trees of a walnut orchard to open the ground for building a superstore.

Pathogenicity Studies
In plant pathology, when we isolate a putative pathogen to make sure that the isolated microorganism is the actual culprit causing the disease, we inoculate the healthy host to reproduce the symptoms. In this case in 2019, we wanted to reproduce again what we observed in the field. A spore suspension of 50,000 spores/ml were prepared, and 10 spurs (three to four fruit) of almonds were inoculated by placing each a drop of 50 µl per fruit or a drop of 100 µl of the inoculum per fruit between the hulls and the shell. Most of the fruit were at the “b3” hull split stage (which is at the deep V split stage, but the nut pops when squeezed.) The nuts of spurs in the 10 shoots that were inoculated with either 50 µl or 100 µl of spore inoculum per fruit showed wilting and blighting of leaves in about one week after inoculation.
Final recording was done 11 days after inoculation when 100% of shoots of inoculated almonds with either 50 or 100 µl/ml of N. dimitiatum were blighted, while the water-inoculated control had 10% blighted and 90% healthy. Re-isolation from the infected fruit produced 100% N. dimitiatum, thus confirming the Koch’s postulates that N. dimitiatum is the pathogen causing this severe hull rot and shoot blight. Cutting the shoots longitudinally one could observe the dark discoloration inside the tissues.
Disease Management
Although no specific studies to control hull rot caused by N. dimitiatum were performed yet, at least we know that the laboratory inhibition studies identified some fungicides that are very effective and should be the first choice for trials in the field. These fungicides included Inspire Super (difenoconazol +cyprodinil, FRAC 3/9), Quadris Top (difenoconazole + azoxustrobin, FRAC 3/11), Orbit (propiconazole, FRAC 3), Quash (metconazole, FRAC3), Luna Experience (flupyram + tebuconazole, FRAC 3/7), Pristine (pyraclostrobin+boscalid, FRAC 7/11), Luna Sensation (fluopyram+ trifloxystobin, FRAC 7/11) and Merivon (pyraclostrocin + fluxopyroxad, FRAC 7/11) (Figure 7).
The fungicides Quash, Orbit and Luna Experience at 10 ppm worked very well in inhibiting the growth of a second strain of N. dimitiatum. The triazole fungicides are also very effective against Aspergillus niger. Another way to manage hull rot caused by A. niger and/or R. stolonifer is to avoid creation of dust during the time the nuts start the hull splitting process. Both of these fungi live in the soil, and the creation of dust brings them in high numbers in the almond nuts. Furthermore, growers need to be aware of the diseases N. dimitiatum causes on other crops (such as branch wilt of walnut, fig limb dieback and canker, and other wilts and cankers in other trees) and avoid pruning or disturbing the infected tissues, thus avoiding spreading airborne spores of this pathogen in almond orchards.