Listen to the audio version of this article. (Generated by A.I.)
Ask a fungus how it evolves, and if it could answer, it would say “adaptation.” Fungi can rapidly multiply and adapt their genes to adverse conditions.
The Eastern Filbert Blight (EFB) fungus, for example, has proved opportunistic and copious. Huge numbers of fungal spores, from a few million to hundreds of billions, can spread and distribute their DNA in record time, on the wind or in rainwater. Despite best practices, including hazelnut trees bred to resist the fungus, it has recently evolved to overwhelm resistance.
The new and virulent race of EFB was discovered in isolated areas of the Willamette Valley two years ago, according to Oregon State University researchers. The new race, a mutated form of the original, has begun its attack on hazelnuts, creating cankers that weaken and may eventually kill the tree.
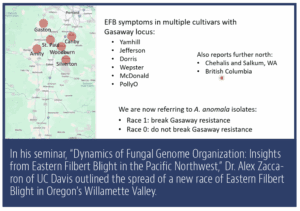
Hazelnuts are not the only plant that have been bred to resist blights, only to face a new race of the same fungus that can overcome resistance. A similar study of a tomato leaf mold, which returned to attack previously resistant plants, is now informing the hazelnut research, according to Dr. Alex Zaccaron of UC Davis, who specializes in fungal genetics. He took a deep dive into the genetics of the new fungal race in his seminar, “Dynamics of Fungal Genome Organization: Insights from Eastern Filbert Blight in the Pacific Northwest,” available online.
Understanding the problem gives hope that it can be solved, he said.
Gasaway-R, the genetic material from a pollinizer hazelnut that in 1975 was discovered to be resistant to the EFB, was a godsend in its time. As the original EFB ravaged Oregon orchards in the 1980s and ‘90s, Oregon State University researchers introduced the first Gasaway genes into seven cultivars and 12 pollinizers. Since 2005, those Gasaway crosses have saved Oregon’s hazelnut crops from the fungal pathogen that has killed more susceptible trees.
Until 2022. That is the year the new race of the fungus, Anisogramma anomala, was discovered. The new race has infected Gasaway-protected varieties including Jefferson, Yamhill, Santiam, Dorris, Wepster, McDonald and PollyO. According to the latest research by Oregon State University’s Dr. Alexandra Weisberg and others, the new race is a mutation of the original pathogen, evolving simultaneously in several orchards in the Northwest. Today, about 60% of the orchards planted to previously resistant Gasaway cultivars are in danger of failing. Very few orchards (most infections are centered in the Woodburn area) actually have the new pathogen, according to Dr. Ken Johnson, a professor of botany and plant pathology at OSU. Still, the infection is concerning.
OSU researchers are now at work attacking the new pathogen from several angles, working on breeding and spraying programs that once again may save Oregon’s hazelnut orchards, and other hazelnut producing states with the potential for this pathogen, from the blight.
History
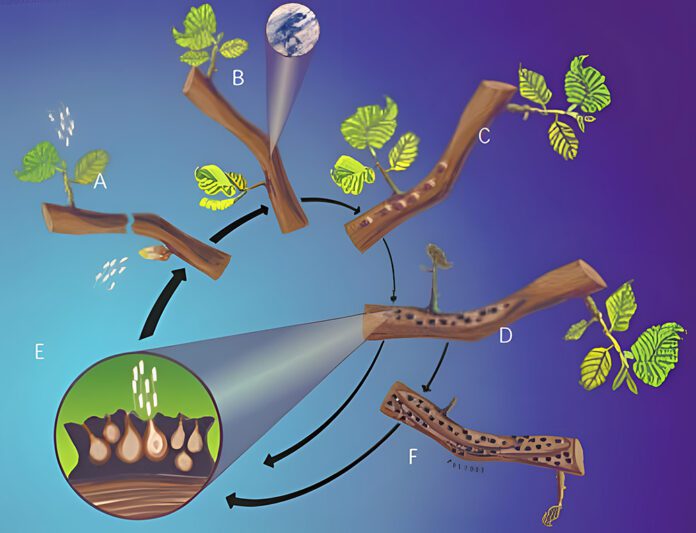
• 1960s: The Eastern Filbert Blight unknowingly first appeared in Northwest hazelnut orchards, possibly arriving on trees from the Great Lakes and Appalachians. Caused by a fungus, A. anomala‘s symptoms include raised, dark-colored bumps or cankers on limbs. Often, the fungus is present for four or five years before symptoms appear. Infected branches die and may eventually kill the tree. In the meantime, the cankers can mature and actively discharge more spores during rainy periods, infecting breaking buds and young shoots.
• 1970s: Surveys revealed how extensive the disease had spread through southeast Washington and threatened the main production areas of Oregon.
• 1980s: The fungus quickly became widespread in the Willamette Valley. In a 1986 USDA study, 30 percent of Oregon’s hazelnut plantings had been affected.
• 1986 to 2009: Over the next two decades, Oregon State University and other research entities developed treatments and several cultivars of trees that proved to be disease resistant. Most Oregon growers have replaced old cultivars with Gasaway-crossed breeds that resist the blight. Thanks to the resistant varieties, Oregon’s orchards, at 23,000 acres in the 1980s, expanded to nearly 100,000 acres today. “Fungicides worked well and gave the breeders time to develop the new cultivars. Otherwise, it would have decimated the industry before deploying the new varieties,” Pscheidt said.
• 2022: EFB is found in an orchard planted to trees thought to be resistant. Researchers renew research on EFB and discover the original fungus may have evolved a new race to bypass the Gasaway resistance.

Research in High Gear
Already experienced at breeding new cultivars resistant to the original blight, OSU researchers are not starting from scratch, Johnson said. Since the 1980s, researchers have studied the behaviors of the blight, mapped its genetics and produced resistant cultivars in a breeding program that continues today. Research has already identified the specific gene they can mark and track in the new pathogen. With that information, they are closing in on the specific genetic material that allows the fungus to evade Gasaway resistance. Because the genes are closely related to the parent, there is hope for growers to control the mutation and possibly breed cultivars with more enduring resistance. Researchers in the Dr. Mehlenbacher and Dr. Rowan labs at OSU are working to breed plants by stacking them with multiple resistance genes in hopes of developing longer-lasting resistance to EFB.
“Just the knowledge that we have. When I started on it in ’88, we didn’t even know how it got in the tree. We didn’t know what kind of materials we could spray on the trees that would prevent infection,” Johnson said.
“…the EFB family tree suggests that new Race 1 had evolved from Race 0 not once, but multiple times, and in different locations in the Pacific Northwest.” – Jay W. Pscheidt and Alexandra Weisberg, Oregon State University
Meanwhile, What to Do?
Report
Now that researchers have identified a specific gene associated with the new race, they can use it as a marker to diagnose its presence in orchards. Using a lateral flow field test, much like that used for COVID-19 testing, growers will be able to rapidly identify EFB and determine which race is present.
Growers who suspect EFB in their orchards, on either resistant or nonresistant trees, should prune off five to 10 cankers and send them in to OSU for testing, according to Weisberg and Pscheidt. Any other cankers should be removed and destroyed. Samples can be shipped to: OSU Botany/J. LeBoldus, Room 2503, 2701 SW Campus Way, Corvallis, OR 97331. Please include contact information, location (GPS best), variety, age of planting and estimated percentage of trees infected.
Evaluation of fungicides for management of the blight is available in a handbook online at http://sites.science.oregonstate.edu/bpp/Plant_Clinic/Fungicidebooklet/2022/Hazelnut5.pdf.
Questions about sending samples, or to request a prepaid shipping label, may be emailed to Alexandra Weisberg (Alexandra.Weisberg@oregonstate.edu) or Jared LeBoldus (Jared.LeBoldus@oregonstate.edu).
Treat
Until resistant cultivars can be bred and distributed, experts are warning hazelnut growers to apply fungicides at bud break and then every two weeks over four applications, among other updated treatments. More is not better, according to Dr. Jay W. Pscheidt, who has directed blight research at OSU since the 1980s. Instead of applying more fungicide, curbing the spread of the fungus requires good timing, he said.
“It takes five hours of branch wetness for the spores of this fungus to start shooting out of the black pustules. When forecasts indicate a week or more of dry weather, fungicide applications can stop and wait until significant rain returns,” Weisberg and Pscheidt said. They recommend applying fungicide every two weeks for four applications. Fungicide applications can stop and wait if there is a week or more of dry weather, then resume when significant rain returns in the forecast. Fungicide must be on the tree prior to rain events.
How Did This Happen?
Back to evolution. A thousand monkeys on typewriters, given an infinite length of time, writing the works of Shakespeare? A fungus would scoff at those numbers if they could scoff. Apparently, with their few billion spores, a wet spring and the valley’s southerly spring breezes to spread them to acres of tender young trees in their path, fungus can create a new race in the space of 10 years or so, according to Zaccaron’s research. While the impulse is to blame new infections on outside imports, Zaccaron’s careful analysis of the new race’s genetics indicated the new race is directly related to the EFB fungus right where it was first found in Oregon. Furthermore, it also didn’t seem to spread only from the Woodburn site but may have evolved concurrently in other sites. In other words, not just a thousand monkeys in a room, but billions in 100,000 rooms.
A. anomala‘s new offspring is so closely related, it varies by just 300 DNA base pair differences out of 342 million, highlighting the close relationship between the two races. Thus, it is called a “race” rather than a new species.
“Unfortunately, the EFB family tree suggests that new Race 1 had evolved from Race 0 not once, but multiple times, and in different locations in the Pacific Northwest,” according to Weisberg and Pscheidt.
On the bright side, the small number of genetic differences between Race 0 and Race 1 means that it may be easier to narrow down which changes are leading the charge against Gasaway resistance. Also, different strains of Race 1 have different mutations, but those mutations occur in the same gene. “Altering this one gene in a similar way may enable A. anomala to change how it overcomes Gasaway resistance. Knowing how it overcomes resistance, and how that resistance works, will assist in the breeding of new resistant hazelnut varieties that provide more enduring protection from EFB,” wrote Weisberg and Pscheidt.















