
Rootstocks are a sustainable tool for safeguarding orchards from pests, diseases and abiotic stress as well as altering tree architecture for diverse planting systems (Vahdati et al. 2021). However, the California almond industry is currently facing considerable challenges with pests and diseases as well as extreme climate events (Lobell and Field 2011; Luedeling et al. 2011; Westphal et al. 2019). Unfortunately, most commercial rootstocks fail to provide a wide spectrum of pest and disease resistance and may not be suitable for new production systems in a changing climate. Further, breeding and evaluation of new perennial rootstocks is a considerable time-consuming investment. The ability to genetically modify existing and new experimental rootstocks in a targeted manner will likely increase the ability to deliver new pest- and disease-resistant and climate-resilient rootstocks in a shorter time frame.
Transformation Project
The HORT57 Almond Board of California funded project titled “Prunus rootstock transformation for a changing climate” is focused on developing a plant regeneration system(s) for genetic transformation of new and commercial Prunus rootstocks used in almond production. Genetic transformation systems have the potential to introduce beneficial genes that function to protect trees from pests, diseases and abiotic stress into existing and/or newly bred Prunus rootstocks. In addition, genes that modify plant architecture for different tree density planting systems and reduce management inputs can also be targeted and introduced into rootstocks to increase production. Moreover, developing a Prunus transformation system will provide an opportunity to develop a gene-editing system(s) to further expand the almond rootstock breeding tool kit. Therefore, genetic transformation systems provide a pathway to introduce beneficial genes into existing rootstocks and/or edit genes without having to go through lengthy breeding cycles to improve rootstock performance to meet challenges associated with pest and disease pressures as well as limit the impact of climate change on production.
*Note: The green arrow points to the callus, which formed at the base of the stamen.
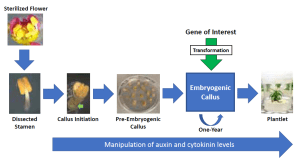
Plant transformation involves the transfer and integration of DNA, including genes, into the genome of a plant cell. Once transformed, a plant must be generated from the transgenic cell(s) (Motte et al. 2014). Therefore, the first step in developing a plant transformation method is to establish a plant regeneration system. When floral organs, leaves and vegetative shoots are removed from a plant and placed in tissue culture on media that induces cell proliferation, dedifferentiated tissue termed ‘callus’ develops at the cut site (Chen et al. 2022). After callus forms, this tissue must be progressed to a state in which plants can be regenerated in an effective manner. At this point, a plant’s regenerative callus cells can be genetically transformed using Agrobacterium tumefaciens. The regeneration of plants from transformed callus cells is achieved by manipulating the levels of auxin and cytokinin, two major hormones that regulate cell division and differentiation during plant development (Ikeuchi et al. 2016; 2013). Generating genetically transformable callus with the capacity to undergo plant regeneration is the primary objective of the HORT57 project.
Regeneration of Prunus rootstocks from somatic or adult tissue has been previously performed from leaves and stems (Gentile et al. 2002; Pérez-Jiménez et al. 2012; Sabbadini et al. 2015). A plant regeneration system termed shoot “meristem bulk” was evaluated for developing transgenic Prunus rootstocks (Sabbadini et al. 2015; 2019). Results showed that while GF677 transgenic plants could be produced at a low rate, most of these selected rootstocks were chimeric, a plant comprised of genetically modified and non-transgenic cells (Sabbadini et al., 2015). In addition, shoot meristem bulk callus derived from Hansen 536 was recently transformed (Sabbadini et al. 2019). However, plants could not be regenerated from the transgenic Hansen 536 shoot meristem bulk callus. Therefore, the regeneration and selection of transgenic plants is a major bottleneck in the development of a Prunus rootstock genetic transformation system.
In the HORT57 project, we are developing an embryogenic plant regeneration system for Prunus rootstock transformation using expertise derived from our grapevine transformation team. To date, we developed an effective method to sterilize floral buds and induce pre-embryogenic callus from the stamens of Hansen 536, Nemaguard, GF677 and Brights Hybrid (data not shown). Currently, we are developing a method to induce embryogenic callus formation, which will be directly used to regenerate plants. If successful, the embryogenic callus regeneration system can be utilized to develop Prunus rootstock transformation system (Figure 1). In addition, we are also evaluating the shoot meristem bulk method to determine if we can increase the plant regeneration efficiency of this system.
The authors would like to thank Almond Board of California for funding the HORT57 project and Mandy Walker for developing the project proposal.
References
Chen, Z., Debernardi, J.M., Dubcovsky, J., Gallovotti, A. (2022) Recent advances in crop transformation technologies. Nature Plants 8: 1343-1351.
Gentile, A., Monticelli, S., Damiano, C. (2002) Adventitious shoot regeneration in peach [Prunus persica (L.) Batsch]. Plant Cell Reports 20: 1011-1016.
Ikeuchi, M., Sugimoto, K., Iwase, A. (2013) Plant Callus: Mechanisms of induction and repression. Plant Cell 25: 3159-3173.
Ikeuchi, M., Ogawa, Y., Iwase, A., Sugimoto, K. (2013) Plant regeneration: cellular origins and molecular mechanisms. Development 143: 1442-1451.
Lobell, D.B., Field, C.B. (2011) California perennial crops in a changing climate. Climate Change 109: 317-333.
Luedeling E., Girvetz, E.H., Semenov, M.A., Brown, P.H. (2011) Climate change affects winter chill for temperate fruit and nut trees. PLoS One 6(5): e20155
Motte, H., Vereecke, D., Geelen, D., Werbrouck, S. (2014) The molecular path to in vitro shoot regeneration. Biotechnology Advances 32: 107-121.
Pérez-Jiménez, M., Carrillo-Navarro, A., Cos-Terrer, J. (2012) Regeneration of peach (Prunus persica L. Batsch) cultivars and Prunus persica x Prunus dulcis rootstocks via organogenesis. Plant Cell Tissue Organ Culture 108: 55-62.
Sabbadini, S., Pandolfini, T., Girolomini, L., Molesini, B., Navacchi, O. Peach (Prunus persica L.). In Agrobacterium Protocols; Wang, K., Ed.; Springer: New York, NY USA, 2015; Volume 2, pp. 205-215.
Sabbadini, S., Ricci, A., Limera, C., Baldoni, D., Capriotti, L., Mezzetti, B. (2019) Factors affecting the regeneration, via organogenesis and selection of transgenic calli in peach rootstock Hansen 536 (Prunus persica x Prunus amygdalus) to express an RNAi construct against PPV virus. Plants (Basel) 8:178.
Westphal, A., Maung, Z.T., Doll, D.A., Yaghmour, M.A., Chitambar, J.J., Subbotin, S.A. (2019) First report of peach root-knot nematode, Meloidogyne floridensis infecting almond on root knot nematode resistant ‘Hansen 536’, ‘Brights Hybrids 5’ rootstocks in California, USA. Journal of Nematology 51: 1-3.















