I recently spent a few days with some great farmers, consultants, manufacturers and scientists at the Healthy Soils Summit in Sacramento. Lots of collaboration and discussion always changes your perspective. It’s a constant battle, this process of trying to manipulate the perfect soil for our trees in an intensive farming environment. Between mono-cropping, herbicides, pesticides, fertilizer, water management, physical soil manipulation and good ol’ Mother Nature, our attempts to solve problems often create many, many more. The emphasis was on data at the conference with many references to the phrase, “If we can measure it, we can manage it.” Which brings us to the question, do we measure our soils correctly, and what does that mean?
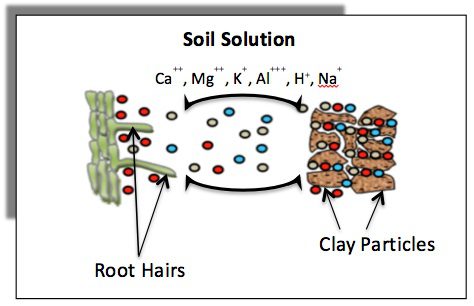
Let’s look at the CEC and base saturation of our soils. To grossly oversimplify things, cation exchange capacity, or CEC, is a measure of how many negative charges exist in our soil that can grab on to those precious earth metals we so voraciously covet: calcium, potassium, magnesium and the snake in the grass, sodium. If the soil holds them, the plant will have a chance to eat them before they leach through the root zone. But often we overlook those other metals that also latch on to our soil colloids like zinc, iron, manganese, and an amazingly detrimental metal in aluminum. I have read published studies that refute our beloved CEC numbers as grossly overstated if we don’t account for aluminum in that test. So, is our measurement actually that accurate? And does that matter? If you are conducting your annual soil test at the same time, in the same places with the same moisture content year after year, you have a baseline you can measure, so you are ahead of the curve. Let’s focus on that and leave the AAc, Bray and Mehlic test arguments to the brainiacs that have the time to analyze them.
Measuring the Soil
I’ll start with my favorite, calcium. A typical soil sample on the west side of the valley in central California would show a 4000-ppm analysis of calcium in our soil after an ammonium acetate extraction method. What does that mean?
Soil weighs on average two million pounds per acre every six inches. Let’s just assume feeder roots reach a depth of 12 inches into the soil. 4000 ppm x 2 (since soil weighs two million pounds per six inches, not one million) x 2 since we are a foot deep and not just six inches, that’s 16,000 pounds of calcium that should be in our soil to one foot deep! Let’s take that a step further to really blow your mind. That’s just the amount that was released when that sample of soil was put through the acetate extraction test. That is not the absolute number. Ron Helland of Sobec ran an experiment with hydronium acid where he rinsed soil 12 times with a low-pH solution until he saw a significant reduction in the number of cations in the extracted solution. In speaking with Joe Mullinax at Denele Analytical, he told me the acetate extraction method was used to more closely mimic what the soil should release in a single year. And now for my favorite phrase when writing these articles for you folks is, “So what?!”

So What?
We can manage it, we can track it and we can adjust what we are actually trying to do about it. If there’s 16,000 pounds of calcium to one foot of soil, or on a 200-ppm test of potassium, 800 pounds K that should be released, why are we ever deficient? In the words of the late great Paul Harvey, “and now, the rest of the story.”
Shouldn’t we be more interested in the soluble component coming out of the water? Don’t we irrigate with water? Not pure water (mind you) and ammonium acetate reacted with potassium chloride, we use water. Let’s change things up and look at it from a different perspective. After a glance at the CEC and base saturation of our soils, look at the water extraction portion in meq/l. That will give you a better idea of what is coming out of the water. The water is what the roots are trying to drink those nutrients from. Adjust the soluble portion of your fertilizers to manipulate what the roots are rinsed with when you feed them. If we have the potential for 800 pounds of potassium to solubilize in a given season just from our soil, that tells me we are leaching a significant portion of it through the roots.
Adjust your farming practices to fertigate differently than you irrigate. Shorter sets are critical when you are applying nutrients. Keep that liquid gold in the root zone as long as possible. When you “measure” your inputs, do the math as well. If a fertilizer is 32% nitrogen and it weighs 10 pounds per gallon, that’s 3.2 pounds N per gallon. A 15-gallon slug of fertilizer may not seem like a lot, with thousands of gallons of water per acre, for 150 trees per acre. However, CCA of the Year Keith Backman, after decades of experience and dedicated service to our farming community, will tell you his lab calculates those trees can only take up 10 units of N per acre per week. Why would we put on 48 pounds N in one shot? If those nutrients keep pushing lower at every irrigation, by the fourth week’s irrigation, how far is the potential remaining fertilizer below the feeder roots where it can’t be absorbed? Do the same for the other nutrients we are calculating to match our trees’ demands at different growing periods.
Does it take more work? Yes. Will it save us more money? Absolutely. Can we make higher yields with less? I am very confident of that and see it all the time with the growers I consult for. Measure it. Know what you are measuring and make your calculations. Apply it in smaller shots more often with shorter irrigation sets. Even an extra day in the root zone can make a difference in the amount absorbed. With less money spent on inputs and more money captured in yields and soil health, you’ll be able to measure that on your bank statements.










